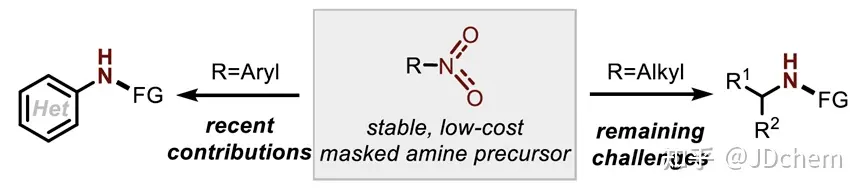
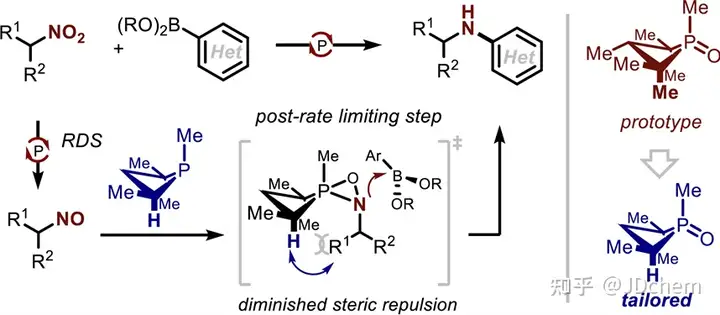
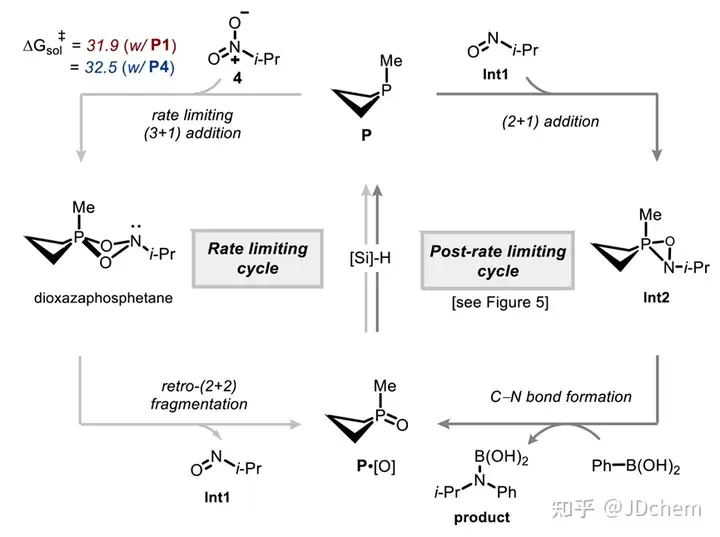
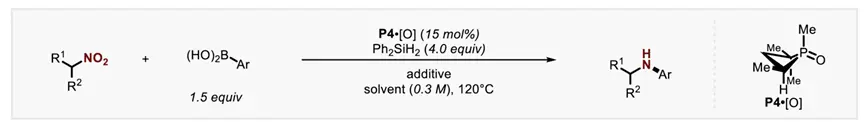
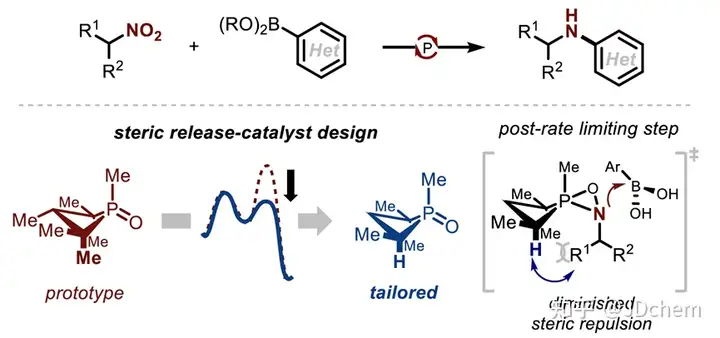
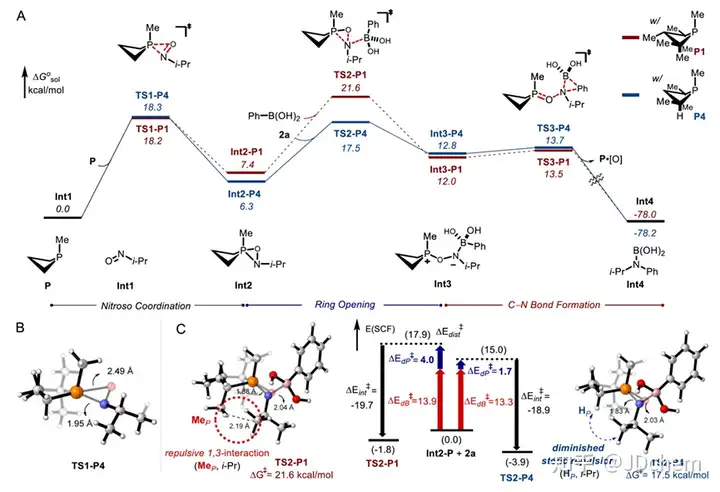
The use of nitro compounds as reagents for catalytic reduction of C-N coupling reactions is an important reaction strategy for direct N-functionalization in the near future. In contrast, the development of catalytic reducing N-functionalized nitroalkanes has been greatly reduced. However, nitroalkanes, as convenient and easy-to-handle amine precursors, are a good research application for C-N coupling chemistry. So, recently Professor Alexander T. Radosevich in J. Radosevich's lecture on J. Radosevich. Am. Chem. Soc. published a selective C-N bond formation reaction between nitroalkanes and aryl boronic acids by catalytic conversion of P(III)/P(V)=O. This redox cycle, facilitated by the P(III)/P(V)=O conversion, drives the coupling reaction between highly functionalized nitroalkanes and aryl boronic acids. The method exhibits excellent chemoselectivity for nitro/boric acid substrate pairs and enables the synthesis of functionalized N-(hetero)aryl amines. This phosphine-based organophosphorus catalyst makes a seemingly peripheral change during the reaction, significantly improving the effect of C-N coupling by preserving the properties of the amphiphilic electronic structure (i.e., the HOMO-LUMO gap). The study also explored the space for coupling in the P(III)/P(V)=O redox process, and found that the reduction of the phosphine catalyst during the reaction was the key to achieving the reaction process. In addition, their combined experimental kinetics and computational studies showed that the sterically reduced catalyst influences the post-rate-limiting step to prioritize the C-N coupling process over other side reactions.
Tel:+86-18271874579
Mobile:+86-18971104330
E-mail: sales@JDchem.com.cn
Whatsapp: +86-18271874579

